
A modern option for your patients
A COMPELLING BODY OF EVIDENCE
Clinical investigators are conducting research to expand the body of evidence to help advance sleep science. Data from our ongoing randomized, double-blind, placebo-controlled^ clinical trial will make important contributions that help clinicians make data-driven treatment decisions. Seventy participants are enrolled in the trial (NW101002), and the data used for Breakthrough Device designation and clearance for marketing was based on 63 participants. Patients in the sham group wore the device, but no vibratory stimulation was provided. The evidence demonstrated the probable benefits outweighed the probable risks.
The clinical trial was designed to enroll four hundred eighty (480) potential participants, two hundred forty (240) adults (120 in each arm), equal to or over the age of 22 years with a diagnosis of PTSD and nightmares. The study randomly assigned participants in a blinded manner to the Active or Sham group. This study was conducted at the Minneapolis Veterans Affairs Health Care System. The primary objective of this trial was to demonstrate that the Active System improves sleep quality as assessed by the Pittsburgh Sleep Quality Index (PSQI) in subjects with PTSD, and that this represents a statistically significant improvement over the change seen in subjects receiving the Sham System.
SAFETY RESULTS
EFFICACY RESULTS
The primary measure of relative efficacy was the difference in mean PSQI change between NightWare and sham arms. The difference observed between the active and sham groups was 1 point.* A secondary measure of efficacy was the change in PSQI-A, which was more than twice that of the sham condition.*
The PSQI-A change was 3.3 points, more than double that of the sham condition (1.4 points). Given that this measure specifically indexes sleep disturbances associated with PTSD, the strength of this dissociation provides evidence that trauma-related nightmares are effectively targeted by the NightWare device.
In an open-label pilot study of NightWare in nine subjects, there was a large effect size of a 6-point improvement in the Pittsburgh Sleep Quality Index (PSQI), a well-validated measure of sleep quality. (Figure 1).
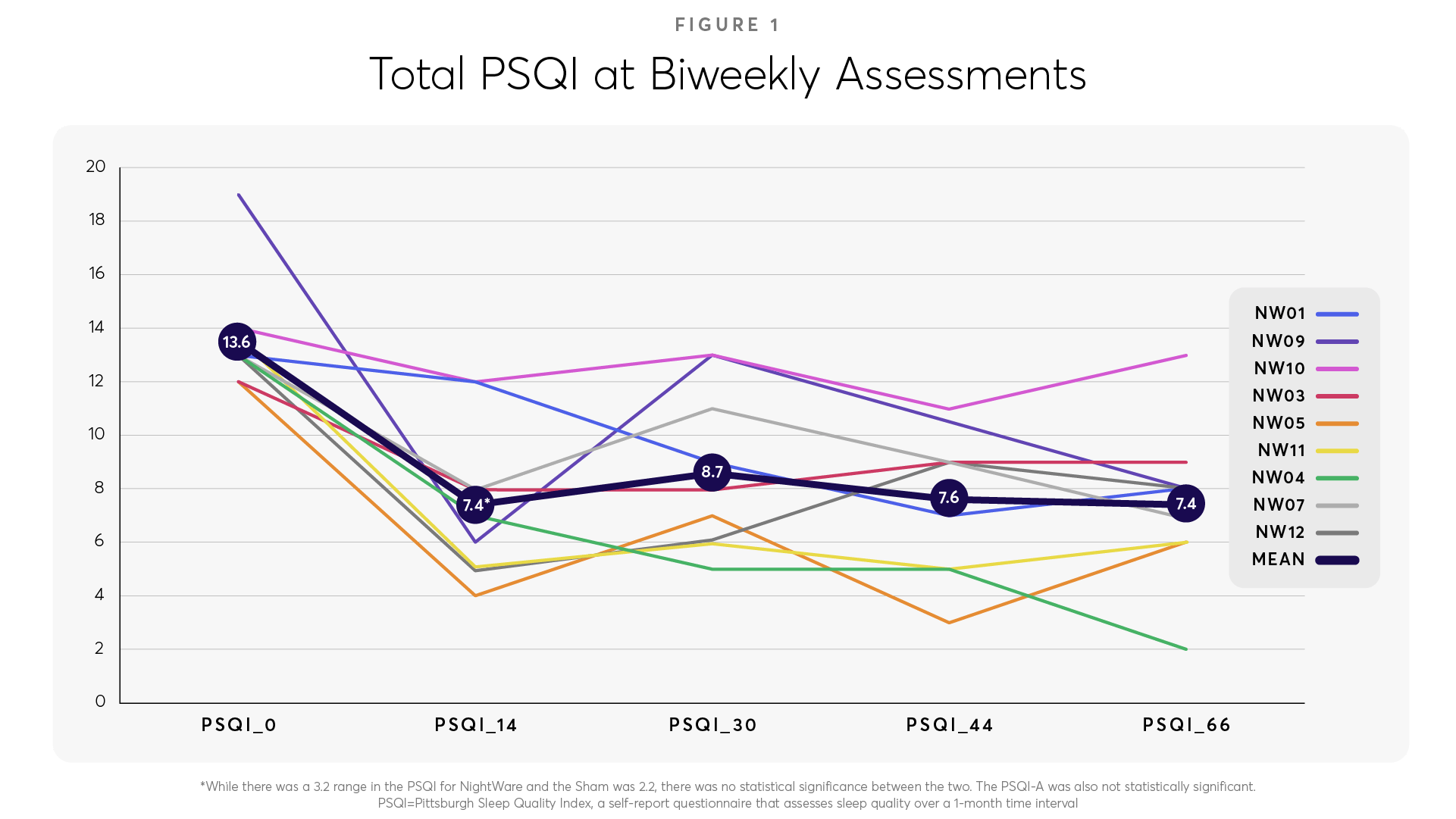
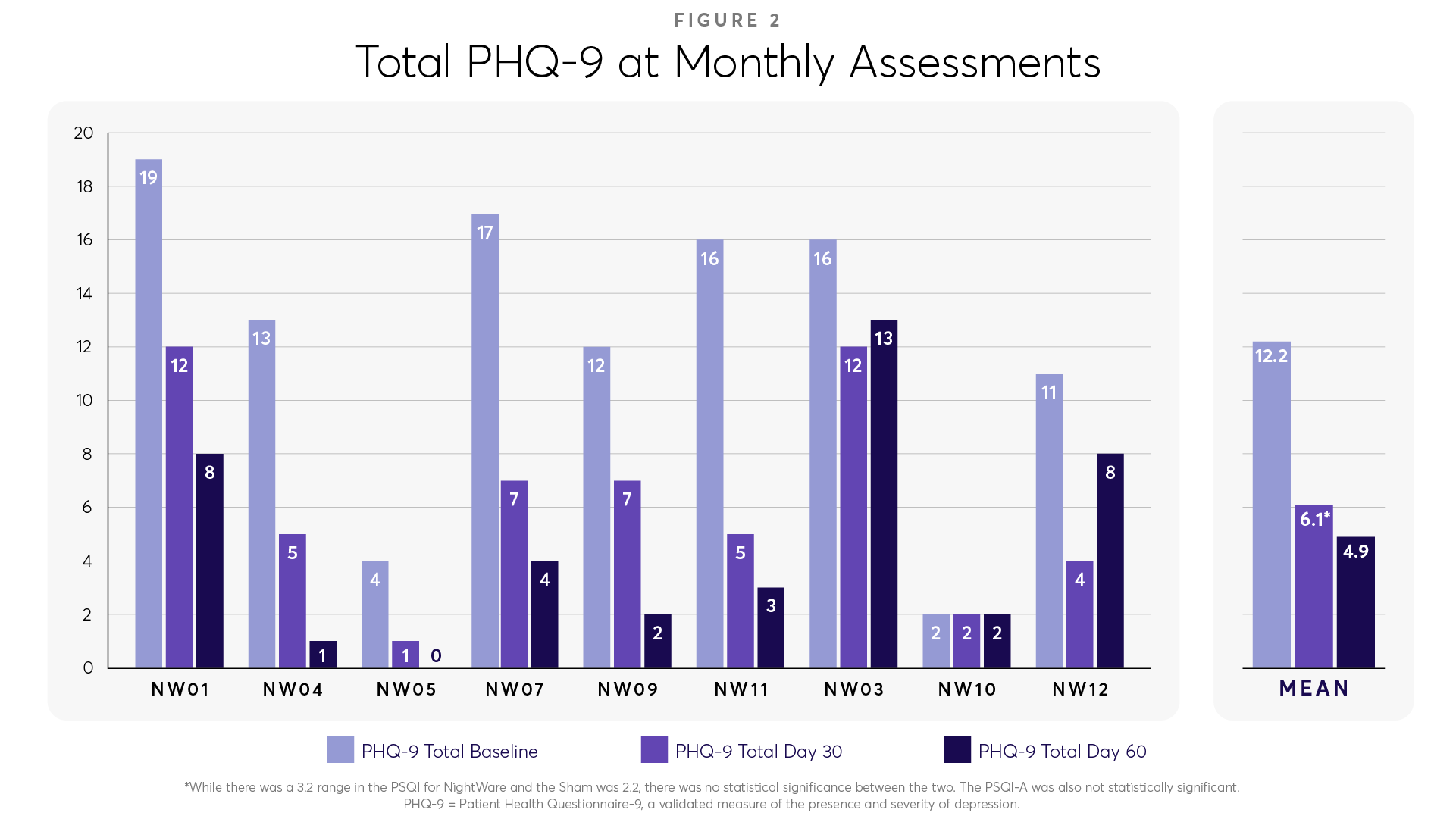
Additionally, the Patient Health Questionnaire 9 (PHQ-9), a standard questionnaire to measure the severity of depressive symptoms, demonstrated a 6-point improvement (Figure 2).
Clinical investigators are conducting three additional studies to generate a larger body of evidence of our prescription digital therapeutic device.
DO YOU HAVE A PATIENT WITH UNDIAGNOSED NIGHTMARE DISORDER?
Millions of Americans suffer from dysfunctional nightmares, and yet they are an underdiagnosed condition.14 A recommendation published in Current Psychiatry Reports suggest, “As nightmares are infrequently self-reported, even in trauma survivors, all patients with a history of trauma should be queried about symptoms consistent with trauma associated sleep disorder.”15
As listed in the DSM-5, repeated awakenings with recollection of terrifying dreams, usually involving threats to survival, safety or physical integrity, is one of the primary symptoms of Nightmare Disorder.16
Other key facts:
- Two large population-based studies put the number of people with frequent, disturbing nightmares at around 2–8% of the population17,18
- About 72% of patients with PTSD have comorbid nightmares19
- Frequent and severe nightmares result in sleep disturbance, which is linked to several psychiatric problems, most notably an association with mood disorders, sleep-disordered breathing, daytime dysfunctional behaviors, and an increased risk of suicide attempts20
- Nightmares may also exacerbate underlying psychological distress in people with depression and anxiety, leading to poor occupational and/or social functioning12,13,14

Looking for an effective treatment for patients haunted by nightmares?
We recognize that many doctors become extremely frustrated when they hit a wall with their nightmare disorder patients and have run out of options. Perhaps you wished there were additional options you could offer to improve the quality of sleep for your patients.
NightWare is not a drug. You can add it to treatments you prescribe for nightmares without concern for interactions.